As we move into Summer in Australia, the world is gearing up to start distributing the Covid-19 vaccine.
Our analysis from Longview Economics in London.
The UK is set to approve the Pfizer vaccine as early as this week, with rollout beginning by 7th Dec. Hospitals in England have been told to prepare for the rollout of a coronavirus vaccine in as little as 10 days’ time, with NHS workers expected to be at the front of the queue. Likewise, Germany expects vaccine approval by mid-December, and the US FDA is meeting on Dec 10th to discuss emergency use of Pfizer’s vaccine.
- Dr Scott Gottlieb, a former commissioner for the US FDA, said that at least a third of the US population will have been infected by coronavirus by the end of the year. Some US states, though, could have infections as high as 50% such as North and South Dakota. Dr Gottlieb has previously stressed that the actual number of infections is likely higher than that suggested by official data, given that not everyone who has contracted the virus has been tested. “We’re probably, at best, diagnosing 1 in 5 cases right now, maybe a little bit less than that, so this is at least half a million cases a day, probably more in terms of actual numbers of infection,” Gottlieb said Nov. 6
- Goldman Sachs has forecast that 70% of people in developed markets will have been vaccinated by fall 2021. Within that, they expect slower vaccinations across Europe (vs. the US) given that they are more reliant on vaccines from AstraZeneca and Johnson & Johnson which have been slower to complete trials.
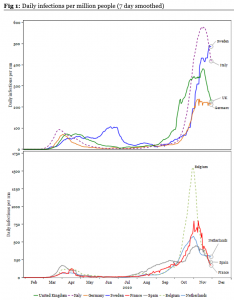
- Key theme: Many European countries are coming out of nationwide lockdowns this week. The key question is how much those lockdowns have reduced the rate of infections within those countries. To answer that question, we have analysed the infection rate among various European countries on a variety of measures. In Fig 1 below, we show that infections per million in most countries is heading lower, albeit remains elevated from levels seen in the summer*.
Where are we getting the vaccine?
- The EU secured 80 million doses of Moderna’s 95% effective vaccine. Under the terms of the proposed agreement, the EC has the option to increase their purchase of mRNA-1273, from 80 million doses up to a total of 160 million doses. The agreement will be finalised following a brief review period by the EU Member States.
- The UK has purchased an additional 2 million doses of Moderna’s vaccine, bringing the UK total up to 7 million from Moderna, and 357 million from 7 different developers.
Chinese vaccine developer Sinopharm has filed for regulatory approval to officially launch its product in China. The company has already vaccinated around 1 million Chinese citizens under a government emergency use program
Vaccine phases explained
Vaccine safety is first assessed in studies with mice or rabbits. If the animals do not develop signs of illness after being given the vaccine, then tests begin with humans in a series of trials which involve steadily increasing numbers:
- After being given the vaccine, then tests begin with humans in a series of trials which involve steadily increasing numbers.
- In Phase I, also called first-in-human trials, the vaccine is given to a small group (10–100) of healthy volunteers. The purpose here is not to test whether the vaccine protects against the disease, but whether it is safe to give – for instance, does it cause a severe reaction? In Phase II, it is given to a larger group (100–1,000), and in Phase III, a larger group still (1,000–100,000).
- Separate studies may be needed in adults, children, and the elderly. Sometimes vaccines that appear to be safe when given to a few people are revealed to have adverse effects when given to thousands, because rarer complications are less likely to be seen when only few people are vaccinated. Continued follow up is important in case of delayed complications. In a pandemic, these sequential studies can be shortened and partially overlapped but it is important to follow thousands of vaccinated people for several months before clearing the vaccine for general use.
General Advice warning: The content of this newsletter is for the clients of Best Interest Advice and it’s other related services. The content is general advice only and has not considered your personal situation or objectives and cannot be relied upon. Please consult a financial adviser to provide you with personal advice. We cannot guarantee the accuracy of this information as it is sourced from third parties and general media. All attempts to verify its contents have been made and we only rely on reputable sources.





